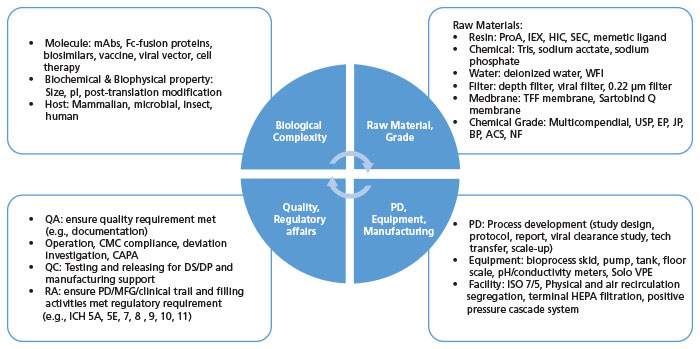
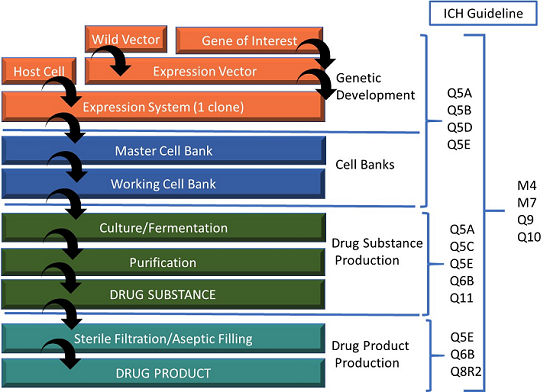
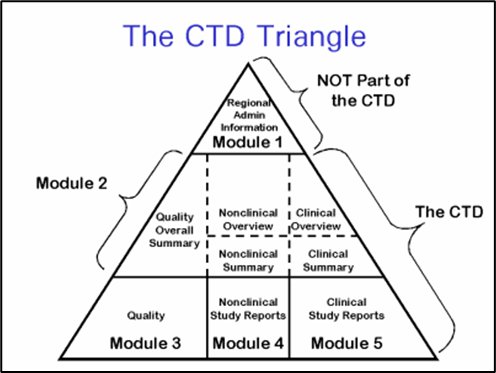
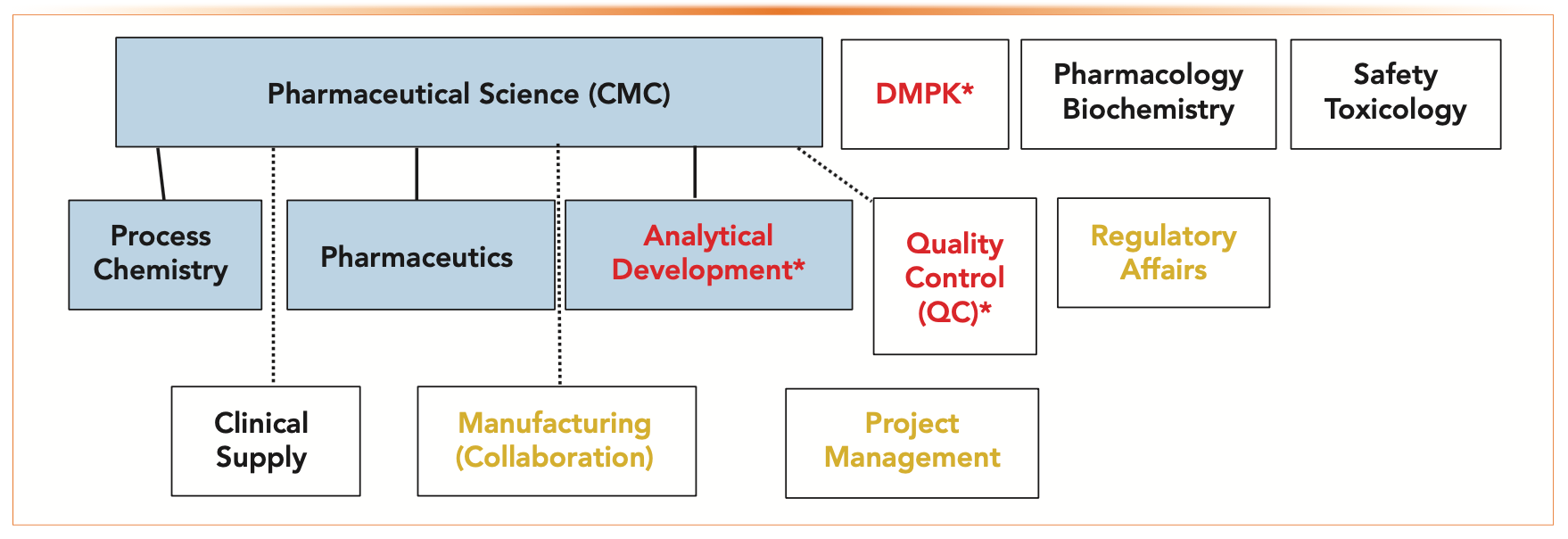
The Future of CMC Regulatory Submissions: Streamlining Activities Using Structured Content and Data Management - ScienceDirect
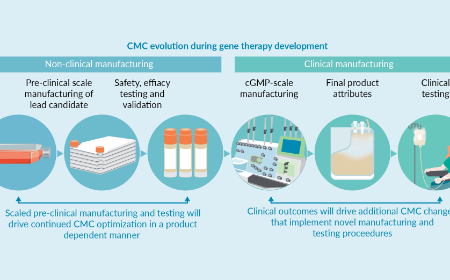
BioInsights - The Chemistry Manufacturing and Controls (CMC) section of gene therapy-based INDs: overview in a changing landscape

Continued Process Verification: Evolution of Biopharmaceutical Control Strategy - CMC ForumBioProcess International
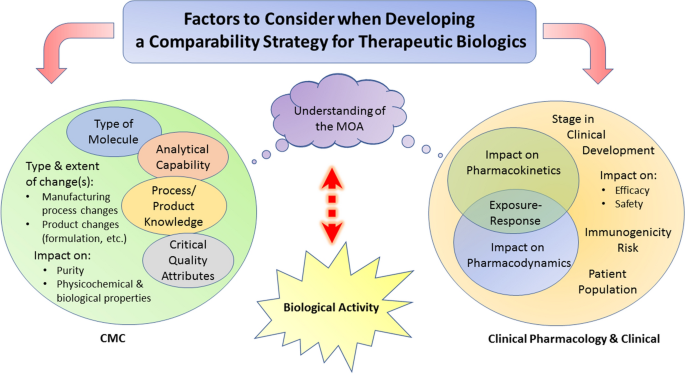
Comparability Considerations and Challenges for Expedited Development Programs for Biological Products | SpringerLink